Researchers at Carnegie Mellon University (CMU) are using ice 3D printing to address a critical challenge in tissue engineering.
Led by a graduate student named Feimo Yang, working under professors Philip LeDuc and Burak Ozdoganlar, the team achieved remarkable precision in fabricating blood vessel-like structures, addressing a key challenge in tissue engineering by replicating intricate microscale networks essential for organ functionality.
According to the team, the pressing need for solutions in tissue engineering is underscored by over 100,000 Americans waiting for transplants and thousands losing their lives, yearly on the waitlist. One of the primary challenges in tissue engineering has been replicating the intricate complex vessel networks vital for organ functionality. Matching the complexity and functionality of natural vessels has proven challenging for traditional artificial blood vessel designs, posing a significant barrier in the field.
In 3D ice printing, a cold surface is typically utilized onto which a stream of water is added. “What makes our method different from other kinds of 3D printing is that instead of letting the water completely freeze while we’re printing, we let it maintain a liquid phase on top. This continuous process, which is what we call freeform, helps us to get a very smooth structure. We don’t have a layering effect typical with many 3D printing,” Yang explained.
A chilling approach to tissue engineering
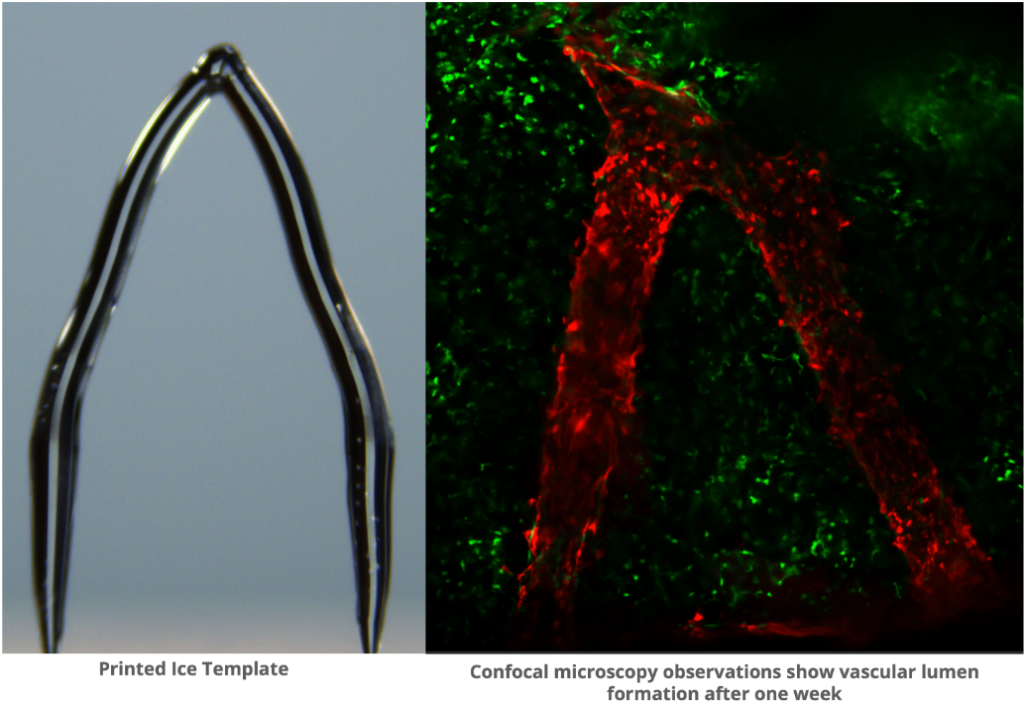
At the core of CMU’s approach lies heavy water, a unique form of water in which hydrogen atoms are replaced by deuterium, raising the freezing point and creating smooth ice structures. These 3D printed ice templates are then embedded in a gelatin material called GelMA.
Upon exposure to UV light, the gelatin solidifies while the ice melts away, leaving behind intricately detailed blood vessel channels that closely mimic their natural counterparts. This breakthrough not only demonstrates the feasibility of creating lifelike blood vessel networks but also opens doors to a myriad of potential applications.
One of the most significant achievements of this research is the successful introduction of endothelial cells, mirroring those found in natural blood vessels, into the fabricated channels. These cells exhibited viability on the gelatin substrate for up to two weeks, hinting at the possibility of longer-term culturing in future iterations.
Beyond its implications for organ transplantation, this approach holds promise for various applications, including drug testing on blood vessels and personalized medicine, according to the team. By coating the 3D printed vessels with a patient’s cells, researchers could also assess how these cells respond to drug treatments, potentially enhancing treatment efficacy and safety.

In the past, CMU researchers developed a method for 3D printing microscale ice structures as sacrificial templates for intricate channels. The process involved jetting water droplets onto a freezing platform, creating smooth ice sculptures that can be embedded in resin and melted away, leaving complex internal pathways. This technology enables devices with liquid or airflow conduits, suitable for soft robots, flexible electronics, and biomimetic human tissues.
Microscale 3D printing for enhanced medical treatments
This year in January, Massachusetts Institute of Technology (MIT) researchers developed 3D printed self-heating microfluidic devices for affordable disease detection. Leveraging multimaterial 3D printing, they customized devices with specific heating profiles. With materials totaling just $2, this cost-effective method enabled deployment in remote areas lacking lab equipment.
By incorporating modified PLA infused with copper nanoparticles as a resistor, they achieved fluid heating during flow. While facing challenges such as temperature limits and fluid leakage, plans encompass integrating temperature sensing and exploring magnet use for particle sorting to refine the technology’s practical applications.
Back in 2021, researchers at the University of Bristol introduced an affordable and open-source 3D printing method for creating microfluidic devices, specifically using PolyDimethylSiloxane (PDMS). By leveraging standard domestic equipment and a desktop 3D printer, the method significantly lowers costs and boosts accessibility in microfluidics research. Its potential impact extends to fields such as laboratories-on-a-chip, cell biology, and protein crystallization, offering promising opportunities for affordable point-of-care diagnostic technology.
Elsewhere, Stevens Institute of Technology researchers leveraged computational modeling to improve microfluidics-based 3D bioprinting, to fabricate complete human organs. Unlike extrusion-based bioprinters, their microfluidics approach allows for precise manipulation of liquids through tiny channels, facilitating the creation of structures as small as tens of microns.
Achieving cellular-scale precision was essential for accurately replicating biological features. According to the team, microfluidics is key in surpassing 3D bioprinting limits, potentially bolstering organ transplants with complex tissue engineering and direct skin application on wounds.
What 3D printing trends do the industry leaders anticipate this year?
What does the Future of 3D printing hold for the next 10 years?
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows 3D printed ice template of blood vessels shown on the left. The right shows imaging of cells forming a blood vessel-like structure on the template one week later. Photo via CMU.



